Biobased Thiol-ene Networks with High Optical Transparency and Abbe Number Derived from Citric Acid
Introduction to Biobased Polymers and Their Optical Applications
Biobased polymers are gaining attention for their potential to replace fossil fuels and address environmental issues. They offer an eco-friendly alternative to petroleum-based plastics and unique functionalities. Transparent polymers are preferred in automotive lighting, optical lenses, and ophthalmic uses due to their lightweight and tunability. High refractive index (n) and Abbe number (νD) polymers are needed for thinner lenses with lower chromatic aberration, though traditional polymers often face a trade-off between these properties. Additionally, they must have high thermal stability, glass transition temperature (Tg), modulus, and strength.
Recently, Professor Chengcai Pang’s team at Tianjin University of Technology synthesized a high-rigidity tetraene compound (4V) from citric acid, then methylated it to produce 4MV with improved rigidity and thermal stability. They developed two thiol-ene networks from 4V/4MV using solvent-free crosslinking with multifunctional thiol monomers. These networks showed high transparency (>90% light transmittance), with refractive indices between 1.5075 and 1.5779 and Abbe numbers from 101 to 235. The study suggests these materials are promising for LED encapsulation, optical coatings, and displays. The work is published in Macromolecules under the title "Biobased Thiol-ene Networks with High Optical Transparency and Abbe Number Derived from Citric Acid."
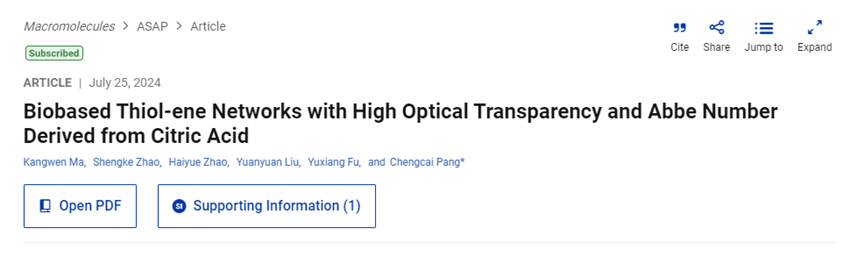
Synthesis and Methylation of 4V and 4MV from Citric Acid
Using citric acid, glyoxal, and 1,3-acetonedicarboxylic acid dimethyl ester, the team prepared a bicyclic tetraester E4 via the classical Weiss-Cook condensation reaction. Subsequently, E4 underwent a solvent-free ester exchange reaction with methanol to yield 4V. Due to the β-keto ester structure of 4V, its thermal stability was relatively poor compared to ordinary carboxylate esters. Therefore, 4V was further methylated using excess CH3I to produce 4MV with near-quantitative yield, improving its thermal stability.
The 1H NMR spectra of 4V and 4MV showed characteristic peaks indicating the presence of enol forms of carbonyl groups and olefinic groups. ATR-FTIR spectra and single-crystal X-ray diffraction further confirmed the successful synthesis of 4V and 4MV.
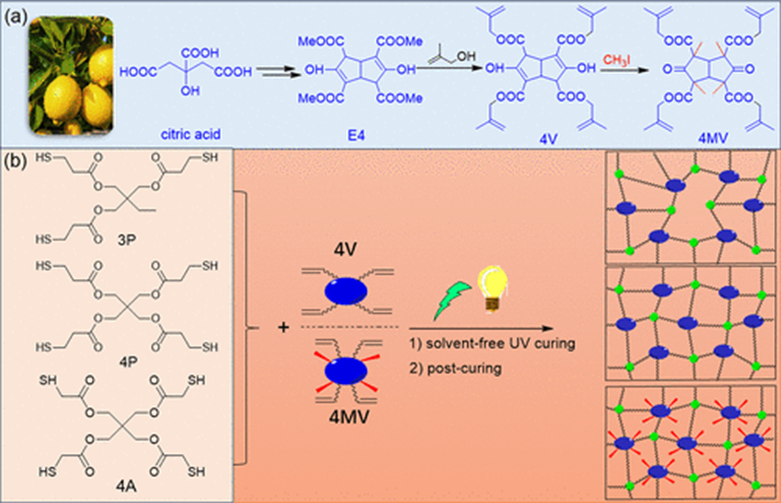
Development of Thiol-ene Networks Through Solvent-Free UV Curing
In this study, the authors selected 4P and 3P as thiols with lower activity and 4A as a thiol with higher activity. 4V and 4MV were crosslinked with 3P, 4P, and 4A in the melt state using DMPA as a photoinitiator in a solvent-free UV curing process, resulting in six thiol-ene networks named 3P-4V, 4P-4V, 4A-4V, 3P-4MV, 4P-4MV, and 4A-4MV. ATR-FTIR spectra indicated that weak absorption peaks characteristic of methallyl groups were still observed at 903 cm-1 and 740 cm-1, suggesting incomplete conversion of functional groups possibly due to the high rigidity and tetra-functionality of the 4V/4MV units.
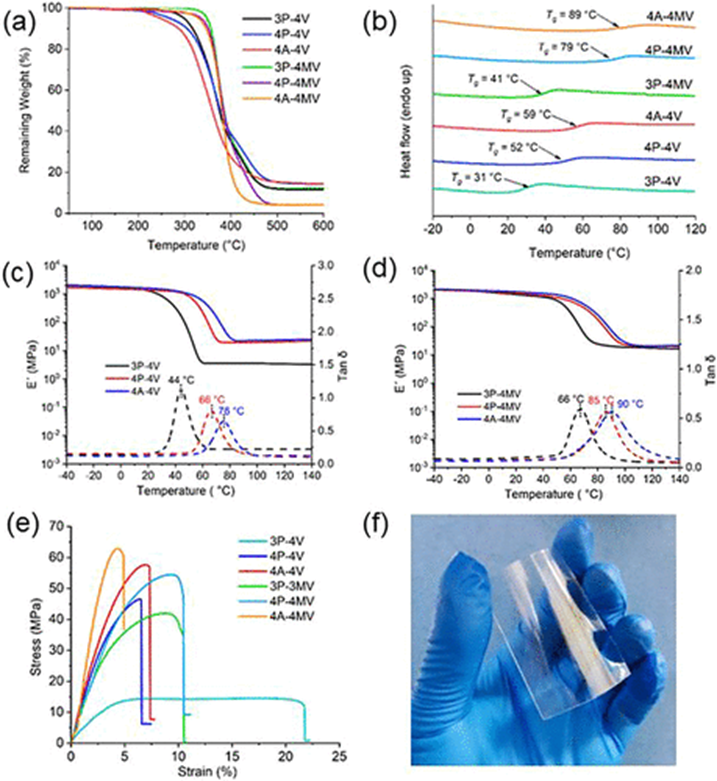
Thermal and Mechanical Properties of the Thiol-ene Networks
Thermal stability of the networks was assessed using TGA, revealing Td5% values of 299°C, 269°C, and 249°C for 3P-4V, 4P-4V, and 4A-4V, respectively. Methylation improved stability, increasing Td5% by 46°C, 65°C, and 59°C for 3P-4MV, 4P-4MV, and 4A-4MV. DSC showed Tg values of 31°C, 52°C, and 59°C for 3P-4V, 4P-4V, and 4A-4V, which rose to 41°C, 79°C, and 89°C for the 4MV series, with higher values indicating increased crosslink density.
DMA testing indicated that storage modulus (E') decreased with temperature, with a single peak on the tanδ curve, reflecting DSC results. The 4MV series showed a storage modulus of 1.38-1.74 GPa at room temperature, comparable to other high-rigidity networks, and demonstrated excellent dimensional stability. Tensile tests revealed smooth, transparent films; 3P-4V exhibited high elongation, while 4P-4V and 4A-4V, with higher crosslink density, showed improved mechanical properties and a glassy state. These properties are due to the rigidity of the 4V/4MV framework and high crosslink density, which restrict chain flexibility and prevent segment sliding. Other tunable thiol-ene systems could be developed by varying thiol compounds.
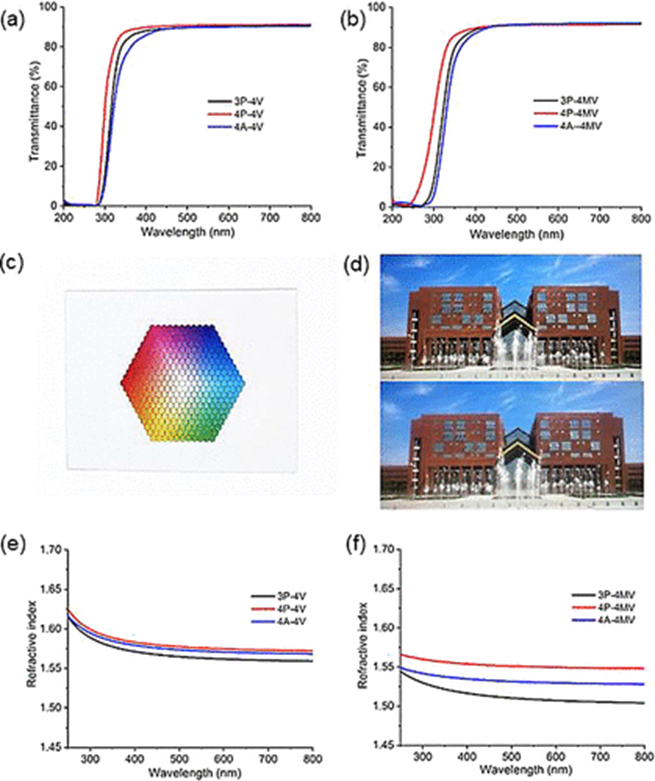
Optical Transparency and Properties of the Thiol-ene Networks
Finally, the authors investigated the optical properties of the thiol-ene networks. The results showed that all films were highly transparent in the visible spectrum (>500 nm) with a transmittance of approximately 90%. This exceptional transparency was attributed to the presence of the alicyclic structure in the 4V/4MV units, which inhibited crystallization and thus enhanced transparency. Moreover, the nD values of all thiol-ene networks ranged from 1.508 to 1.575, comparable to norbornene copolymers and semialicyclic polyimides. All polymers exhibited νD values greater than 100, which might be due to their fully aliphatic structure that reduced near-UV absorption, thereby decreasing wavelength dispersion.
Conclusion and Potential Applications of the Thiol-ene Networks
In this work, the authors synthesized a bicyclic tetraene compound 4V from citric acid, and further methylation produced 4MV with improved rigidity and thermal stability. Using multifunctional thiols, they prepared two types of thiol-ene networks through a solvent-free UV curing method with 4V and 4MV. At room temperature, 3P-4V exhibited rubber-like elastic behavior, while 4P-4V and 4A-4V exhibited higher Tg values and superior mechanical properties due to their dense crosslinked structures. In the 4MV series, methylation improved both thermal stability and mechanical properties, with 4A-4MV exhibiting the highest Tg value and mechanical performance due to its high rigidity and crosslink density. All thiol-ene networks demonstrated transmittance close to or exceeding 90% (>500 nm), moderate n values (1.5075-1.5779), and very high νD (>100), making them promising candidates for various optical materials.



